While in daily clinical practice the TNM staging system is used to predict prognosis and base treatment decision on, the failure of radiotherapy treatment can be attributed to factors on different levels. The treatment, patient characteristics, tumor characteristics and cell properties can all contribute to the eventual cure or failure (summarized in figure 1.7).
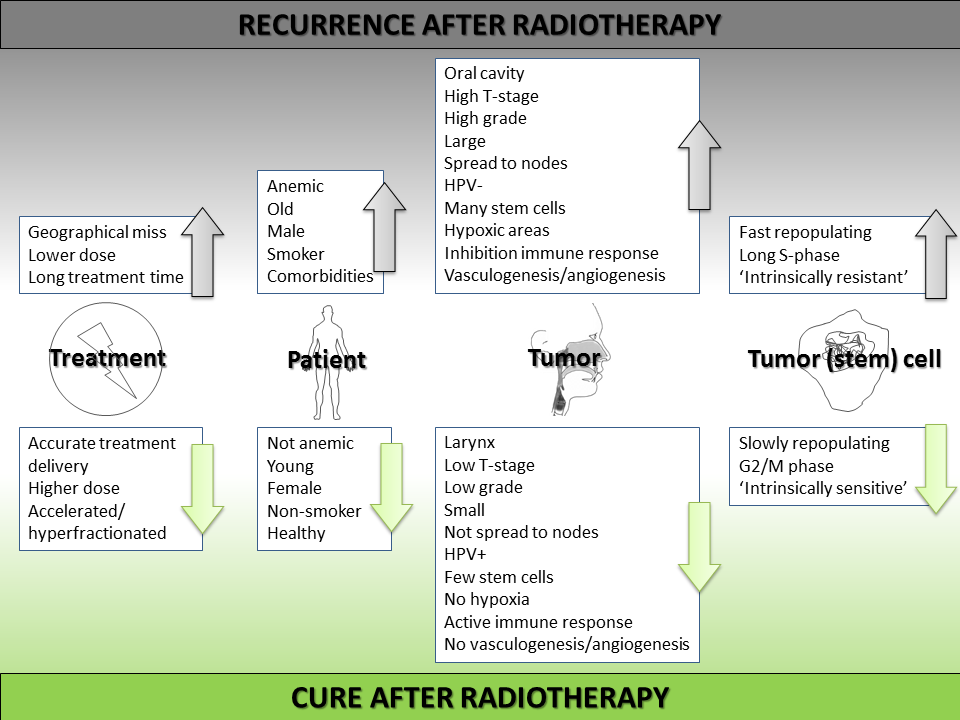
Figure 1.7: Overview of causes of radiotherapy failure (upwards arrows) or success (downwards arrows) in head and neck cancer.
1.3.1 Treatment characteristics
Radiotherapy
Radiotherapy using photons causes damage through the generation of free radicals or through direct damage in the cell. Radiation damage causes various DNA defects, of which the most lethal is the DNA double strand break. An illustration: a typical fraction dose of 2 Gray (Joule/kilogram) induces > 2,000 DNA base damages, ∼2,000 DNA single strand breaks, and 40-80 DNA double strand breaks per cell (82). Every 2 Gy-fraction will kill around 30-50% of the tumor cells. Fractionated radiotherapy uses the principle that normal tissues have a better ability to repair (DNA) damage than tumor cells, and will therefore (partly) recover in between fractions, while tumor cells will not. The relative advantage of a treatment course integrating both the tumor and the normal-tissue effects can be expressed in a therapeutic ratio (41). Alterations in the radiotherapy fractionation or the addition of chemotherapy or targeted therapies aim to specifically target tumor cells and thereby improve the therapeutic ratio. An example of a typical curative head and neck irradiation schedule is 35 fractions of 2 Gy (total dose 70 Gy) over 7 weeks. To make different irradiation schedules comparable in terms of the biological effect, treatment schedules can be recalculated to the equivalent dose in 2-Gy fractions (EQD2) using the Linear Quadratic (LQ) model (Lecture LQ model by Adrian Begg). Assuming the overall treatment time is unchanged the formula is: EQD2 = D * (d + α/β)/(2 + α/β), were D is the total dose, d the dose per fraction and the α/β-ratio represents the fractionation sensitivity of the tissue of interest (for head and neck cancer 10 is commonly used). An EQD2 converter can be found here: EQD2 converter. When the overall treatment time is changed (usually to accelerate the treatment) the EQD2 is calculated as follows: EQD2,t.new = EQD2,t.old – (t.new – t.old) * Dprolif, where t.new is the new overall treatment time in days, t.old the original treatment time in days and Dprolif is the dose recovered per day due to proliferation; for head and neck cancer this is 0.7 Gy/day (83).
Treatment and treatment delivery
Different treatment-related factors can contribute to treatment failure. The total dose (EQD2) has been shown to predict survival (84, 85). A higher total EQD2 gives a better tumor control, but is sometimes compromised because of an interruption of the treatment. Other reasons for a lower EQD2 are concessions due to dose limiting normal tissue toxicity (important but not discussed in this thesis) or missing part of the tumor extent on pretreatment imaging leading to a lower dose or complete geographical miss of the tumor, meaning part of the tumor will not receive the total dose needed for tumor kill. Time plays an important role in head and neck cancer radiotherapy. Both the overall treatment time and the time to treatment initiation have been shown to be important predictors of outcome. A delayed start of treatment is prognostic ally unfavorable (86, 87). Data from the US National Cancer Database show that patients with a waiting time under 52 days have a median overall survival of 72 months, versus 47 months for patients with a waiting time over 67 days (86). Radiotherapy can further be improved by using accelerated (reduction of total treatment time) or hyperfractionated (more fractions in the same treatment time) treatment schedules (72, 79, 88). A lecture by Jack F. Fowler on hyperfractionated and accelerated radiotherapy can be viewed here: altered fractionation (Jack F. Fowler, 1989). The benefit of these regimens is an absolute overall survival benefit of 3.4% at 5 years (8.2% for hyperfractionation). Other strategies to improve radiotherapy outcome are the addition of chemotherapy (89) or targeted therapy (90). Concomitant chemotherapy gives an absolute survival advantage of 6.5% at 5 years in a large meta-analysis (91). On the addition of targeted therapies to radiotherapy, there are no meta-analyses yet. So far, a combination of radiotherapy with the hypoxic sensitizer (nimorazole) (92) or the EGFR-inhibitor cetuximab have shown promise (90). More targeted therapies are currently under investigation, as well as proton therapy (93).
1.3.2 Patient characteristics
Patient-related factors
Many patient characteristics have been described to influence cure and survival rates. Factors that have been linked to decreased survival are a higher age, male sex, pre-treatment anemia, a poor general health/comorbidity and (persistent) smoking. Older patients do worse that younger patients; this has been shown in several studies with an average hazard ratio of 1.5 per decade (84, 85, 94, 95, 96). Male sex was reported to have a hazard ratio of 2.3 compared to female sex in a series of 994 laryngeal cancer patients that were treated with radiotherapy (84). Patients with a low hemoglobin concentration before start of radiotherapy have a worse overall and disease free survival rate, with a hazard ratio of around 1.4 for patients with anemia (84, 85, 97). The worse survival of anemic patients cannot be overcome by a transfusion prior to the start of treatment (98). Patients with a worse general health score either defined as performance status (85, 99), ASA comorbidity score (100) or ACE-27 score (101) do worse than healthy patients without comorbidities (102). In a large study conducted to identify behavioral factors that influence survival of head and neck cancer patients, being a former or current smoker gave a decreased overall survival (with respective hazard ratios compared to never smokers of 2.0 and 2.4) (95). Molina et al. reported a slightly lower hazard ratio of 1.3 for tobacco use (94). In a recent study by Gillison et al. the risk of death increased by 1% per pack-year that was smoked (103).
Tumor-related factors
Tumor properties that have been described to influence cure rates negatively are a higher T and/or N-stage, a large tumor volume, the site from which the primary tumor originates and biological characteristics. A higher T stage is correlated with a worse overall survival, with estimated hazard ratios of 1.5, 2 and 3 for T2, T3 and T4 tumors compared to T1 tumors (73, 84, 85, 96, 97, 101). Another way to describe the primary tumor is the measurement of the primary tumor volume on pre-treatment imaging (CT/MRI/PET). The larger the tumor volume, the worse overall survival rates, this was demonstrated in a few studies that measured tumor volume, the overall survival rate was reported to decrease around 10% for every 10 cm3 volume increase (100, 104, 105, 106, 107). In all of these studies, the addition of tumor volume to a multivariate model eliminated T-stage as a significant predictor of overall survival. The extent of lymph node involvement, the N stage is often correlated with survival, in a study by Schroeff et al. 5 year survival was 61.3% for N0 and 10% for N3. Others have reported similar findings (84, 85, 96, 97, 99, 101, 104), with hazard ratios compared to N0 for respectively N1,N2 and N3 patients, being around 1.5, 2 and 3. Different studies show the importance of tumor subsite for the prediction of outcome (84, 94, 95, 97, 99). A representative example is the study by Schroeff et al., which showed that in a large population cohort, patients with a glottic larynx tumor had a much better 5 year survival (68%) than other sites like oral cavity (42%), oropharynx (37%) or patients with hypopharynx (28%) tumors (101).
1.3.3 Tumor biology
The survival of patients can be influenced by general prognostic biological factors like tumor grade or HPV status, but also by predictive factors that are (partly) specific for the response to radiotherapy (108). The grade of the tumor is a measure for its aggressiveness that correlates with prognosis. In a study by Molina et al. a moderate to poor differentiation grade has a hazard ratio of 1.2 over good differentiation (94, 101). Fairly recently the HPV infection status has been discovered to be a major factor for the prediction of outcome of head and neck cancer (109). Patients with HPV-positive tumors have a reduction in the risk of dying from their cancer when compared with HPV-negative tumors. In a meta-analysis of 37 studies by Ragin et al. a 28% reduced risk of death was observed (hazard ratio 0.72) (110). In three studies of patients treated with (chemo-)radiotherapy a consistent 60% reduction in the risk of death was observed (hazard ratio 0.4) (111, 112, 113). The superior cure rates of patients with HPV positive tumors might be caused by an increased sensitivity to irradiation due to impaired DNA repair (114, 115). Because of their superior survival, patients with HPV positive tumors have even been suggested to be candidates for treatment deintensification (49, 116, 117).
Classical radiobiological processes influencing tumor response to irradiation are oxygenation, proliferation and intrinsic radiosensitivity (118), also described as the 4 or 5 ‘Rs’: Repair, Reoxygenation, Repopulation, Redistribution of cells in the cell cycle and intrinsic Radiosensitivity (119, 120). More recently other processes have been added to these factors: the presence of stem cells, microenvironmental factors like blood supply and immune cells and possibly also the energy metabolism of the tumor cells (121, 122).
Repair
The term repair, or recovery, is often interpreted as ‘DNA repair’, but was originally (before the discovery of DNA repair) used to describe the observation that tissues can recover after radiotherapy. This recovery has different aspects: repair of DNA damage (discussed under ‘intrinsic radiosensitivity’) and tissue factors (discussed under ‘microenvironmental factors’).
(Re-) oxygenation
Hypoxic cells treated with radiotherapy have a survival advantage. This was shown by numerous in vitro studies (among others: (123, 124, 125, 126)). The fact that hypoxia is a negative prognostic factor, has also been shown in vivo, using different techniques to evaluate the level of hypoxia in a tumor (127). Hypoxia can be measured directly by invasive methods or indirectly by imaging techniques or by studying protein or messenger RNA expression of genes known to be involved in hypoxia (127). Of note is that hypoxia is often subdivided into chronic (diffusion limited) and acute (perfusion limited) hypoxia, which of these two has the most implications for therapy outcome is still under debate (128). Many methods to study hypoxia in a tumor exist, consisting of invasive methods, different imaging techniques and various analyses of biopsy material (129). Direct, pre-treatment Eppendorf pO2 measurements with an oxygen sensitive needle probe inserted into the tumor, demonstrated that a high percentage of hypoxic areas within the tumor was associated with poor survival (22, 130, 131, 132). Studies of PET imaging of hypoxia with different tracers indicated that, again, hypoxia correlates with worse control rates after radiotherapy (133, 134, 135, 136). Hypoxia PET scans can also be of use in the monitoring of hypoxia during treatment: a decrease of hypoxic tumors from 70-100% before treatment to 6-36% during treatment was observed (133, 137, 138). Another imaging strategy to study hypoxia is MRI, using specific scanning protocols, like dynamic contrast enhanced (DCE) MRI (129, 139, 140).
The most extensively immunohistochemically studied hypoxia markers are the exogenous pimonidazole and the endogenous markers HIF1-alpha and carbonic anhydrase IX (CAIX). Pimonidazole (an exogenous compound preferentially bound by hypoxic cells) staining correlated with local control after radiotherapy: 2-year local control rates increased from 48% to 87% when pimonidazole staining decreased (141). Overexpression of HIF1-alpha, a proposed marker for acute hypoxia, correlated significantly with worse local control (142, 143, 144), as well as expression (pattern) of CAIX, a HIF-1alpha target and pH regulator (142, 145, 146, 147). With the notion that one marker might not reflect the complex cellular response to hypoxia, there have also been reports of panels of markers (gene expression sets) studied simultaneously that correlate hypoxia status with outcome (148, 149, 150, 151).
Finally, the fact that in vivo modification of oxygen status during radiotherapy can improve local control, especially in hypoxic tumors, proves that hypoxia is an important factor in radioresistance (92, 133, 138, 151, 152, 153).
Repopulation/proliferation (Link to lecture by Adrian Begg on proliferation)
Using fractionated radiotherapy, not just normal tissues, but also tumors have the opportunity to compensate for their loss, meaning fast proliferating tumors will (partly) renew themselves in between fractions. Two factors are of importance for this phenomenon: the ability to proliferate quickly and the number of cells that have clonogenic capacity (154). The potential tumor doubling time, measured on pre-treatment biopsy material, was a significant predictor in single center studies, but failed to show a significant correlation with outcome in a multicenter validation study of 476 patients (155). However, in head and neck cancer, a negative effect of prolongation of overall treatment time has been shown. From about 5 weeks after the start of fractionated radiotherapy an accelerated repopulation has been observed, meaning that with a longer overall treatment time, more dose is needed for the same tumor control rates (156, 157, 158). This observation has been used to design new fractionation schedules. When the same dose (70 Gy) was administered in 6 weeks instead of 7, a significantly higher tumor control rate (around 10% higher) was observed (79, 159). However, not all patients appear to benefit from accelerated radiotherapy, additional subgroup analyses have shown that the benefit is for patients with a well differentiated, slowly proliferating tumors with high EGFR expression (160, 161, 162, 163). An explanation for this counterintuitive finding could be that these tumors resemble normal mucosa and therefore still share the ability for accelerated repopulation (164). Another approach to measure proliferation of a tumor, could be to detect the glucose uptake (165). Tumor uptake of 2-[(18)F] fluoro-2-deoxy-D-glucose (FDG) measured by positron emission tomography (PET) has been shown to be a prognostic factor in a series of 120 head and neck cancer patients. A higher glucose uptake (measured by a higher standardized uptake value) was correlated with worse disease free survival (166).
Redistribution
Over 50 years ago it was observed that cells in different phases of the cell cycle showed different survival rates after irradiation (167, 168). It was shown that cells are generally more sensitive to irradiation during mitosis/G2 phase and more resistant during the (late) S phase. Fractionating radiotherapy would increase the probability of irradiating cells in a more sensitive phase, because of the redistribution in phases in between two fractions (119, 169).
In series of head and neck cancer patients treated with differently fractionated radiotherapy schedules, it was observed that tumors with a longer duration of S phase (measured in vitro) had worse local control rates: around 30-40% in tumors with a longer S phase, compared to 50-60% for tumors with a shorter S phase duration (155, 170).
Intrinsic (cellular) radiosensitivity
Within a tumor, different cell populations exist, with different sensitivity to irradiation (171). Tumor cell radiosensitivity, defined as the sensitivity of cells to ionizing radiation in vitro, is a significant prognostic factor for radiotherapy outcome (118). The sensitivity of cells in vitro can be tested by measuring clonogenic survival at specific doses of irradiation. The percentage of surviving colony-forming cells at a certain dose level can then be determined. Survival of cells at 2 Gy was shown to correlate with tumor control rates in studies that compared in vitro cellular radiosensitivity to therapy response (172, 173, 174, 175). Hypothetical causes for cellular radiosensitivity can be divided into three categories: 1. Cells get less damaged upon irradiation, 2. Cells repair DNA damage better/faster after irradiation, 3. Cells with the same amount of damage have better pro-survival mechanisms.
Although there is not much evidence for the first hypothesis, it has been suggested that cells with more radical oxygen species scavengers, like glutathione, have higher survival rates (176, 177). Another possible factor contributing to the evasion of damage from radiotherapy is chromatin density. Areas of more condensed chromatin have been shown to be less prone to double strand breaks (178, 179). The second hypothesis, better DNA damage repair, is probably the most important and most investigated explanation for intrinsic sensitivity. Cells that are defective in DNA repair are more sensitive to irradiation. This can be learned from patients with DNA repair disorders (180, 181). Luckily, in most cancer patients, impaired DNA-repair is specific to tumors, which leads to improvement of the therapeutic ratio of fractionated radiotherapy. Numerous in vitro experiments have shown a radiosensitization after the inhibition of one of the DNA repair pathways (128, 182, 183). Some drugs targeting the DNA damage response are currently tested in clinical phase I/II studies (184). A lecture on the exploitation of DNA repair by Adrian Begg can be viewed here: Exploiting DNA repair to improve radiotherapy. Finally, the ability to evade death after getting damaged by irradiation could contribute to cells being more resistant. Firstly, by the correct activation of cell cycle checkpoints upon obtaining DNA damage, a cell can take the time to repair damage and thereby evade mitotic catastrophe. There is evidence that cell cycle checkpoint inhibition can lead to higher tumor control rates (185). In case the damage is too extensive, there are many ways for a cell to die (186, 187). Although the most researched method, apoptosis through TP53 signaling, is not consistently linked to radiosensitivity, other modes of dying could be correlated with radiosensitivity (188, 189). There is some evidence in head and neck cancer that TP53 does not inhibit apoptosis, but causes treatment failure by evasion of senescence (190).
Other processes (not starting with an ‘R’)
Since the 4 or 5 classic ‘Rs’ have been defined decades ago, there are some new insights as to why tumors can be radioresistant. Firstly, the discovery that not all cells in a tumor are important for the survival of that tumor gave rise to the characterization of the cancer stem cell model (Lecture Professor Weinberg on cancer stem cells): only some cells in a tumor are able to regrow a new tumor and are therefore the only cells that need to be killed in order not to get a tumor recurrence after radiotherapy (191, 192). This means that all other factors (all classic ‘Rs’) are only important for those cancer stem cells (121). There is a growing body of evidence suggesting that not only the percentage of cancer stem cells in a tumor is important, but that cancer stem cells are intrinsically more radioresistant than non-cancer stem cells (193). Secondly, there is a growing recognition that the microenvironment in which a tumor cell grows is important for its response to irradiation. The microenvironment can influence radiotherapy response in several ways. Cancer cells can be influenced by their neighboring cells, leading to the bystander effect (indirect damage of initially undamaged cells because they are next to irradiated cells) (194). Another important component of the microenvironment is the vasculature. Tumor often have a ‘messy’ vasculature leading to various levels of hypoxia. Additionally, endothelial cells dying as a response to radiotherapy can prevent the regrowth of tumor cells that were being supplied by that vessel (195). The inhibition of vasculogenesis has been shown to prevent tumor recurrence in glioblastoma xenografts (196). Other important cells in the microenvironment are the immune cells. Infrequently an abscopal radiotherapy effect is observed: stimulation of the immune system by irradiation of one tumor location can stimulate immune cells to eradicate tumor cells at an unirradiated site in the same patient (197). Given recent breakthroughs in cancer immunotherapy, there is a growing interest in the stimulation of this abscopal effect by combining radiotherapy with immunotherapy (198, 199, 200, 201, 202). Lastly, the altered energy metabolism of tumor cells can have an effect on radiosensitivity; a different redox state can lead to more ROS scavenging or have an effect on immune invasion or angiogenesis (122, 203, 204).
Prediction of response to radiosensitizers
Biological properties are not only useful to predict response to radiotherapy, but also response to radiosensitizers. It has been shown that pre-treatment tumor hypoxia status can predict benefit from hypoxia-sensitizers added to radiotherapy (92, 133, 138, 151, 152, 153). Response to concurrent cisplatin could be predicted by measuring cisplatin-DNA adduct levels or loss of nuclear p53 signal (205, 206) and a worse response to EGFR inhibitor has been attributed to activation of ERK signaling, KRAS mutations or the absence of the KRAS-variant (207, 208, 209, 210).