It is not clear why some cells are radiosensitive and others are intrinsically radioresistant. By identifying the underlying mechanisms of radioresistance, it should become possible to personalize therapy where necessary, thereby achieving better treatment success rates. In this study, we correlated expression of miRNA and mRNA to intrinsic radiosensitivity of head and neck cancer. In our HNSCC cell line panel, we found that a low expression of certain miRs was strongly correlated with radioresistance. Different analysis methods led to the conclusion that EMT was an important factor in radioresistance, namely, the top correlating mRNAs, miRs, and gene sets were all involved in EMT and these findings were validated by testing two different cell lines engineered to undergo EMT, which caused an increase in resistance. Next, we have shown that low expression of the top miR (miR-203) predicting intrinsic radiosensitivity indeed corresponded to more local recurrences after radiotherapy in a patient series of laryngeal carcinomas. Because it has previously been reported that no major difference was detected in miR profiles among laryngeal, oropharyngeal, or hypopharyngeal cancers, we believe that this cohort could be representable for all of these subsites (36). It should be noted that results were obtained using multiple testing on a small series, needing further validation in a larger cohort of head and neck squamous cell carcinomas, preferably including head and neck tumors from different subsites.
Although separate EMT genes like fibronectin 1, Snail, Slug, and E-cadherin have already been associated with radioresistance (37–40), it has not been clarified why EMT would cause radioresistance. We hypothesize that simultaneous with acquiring a mesenchymal phenotype, the mechanisms by which cells can become more resistant to irradiation are altered. EMT is mainly a description of a phenotype, but the fact that the acquisition of this phenotype is correlated with radioresistance may indicate it affects at least one of the three known mechanisms that lead to resistance: less damage upon irradiation, better repair of irradiation damage, or less cell death upon damage.
A first hypothesis could be that the evasion of DNA damage could lead to radioresistance (31). In a recent overview, Watson proposed that mesenchymal cancer cells possess heightened amounts of antioxidants that reduce damage caused by irradiation-induced reactive oxygen species (ROS; ref. 41). Gammon and colleagues showed that within mesenchymal cancer cells under normoxic conditions, a subpopulation of cells with low oxygen and ROS levels can be found (42).
Second, a more effective DNA damage repair system can lead to increased survival of cells after radiotherapy. This appears to be the case in breast cancer cell lines, in which it was shown that HOXB9 induces both EMT and confers resistance to ionizing radiation by accelerating the DNA damage response (43). In another report, it was shown that ATM-mediated Snail serine 100 phosphorylation regulates cellular radiosensitivity (44).
Finally, damaged cells can evade cell death and thereby survive irradiation. Kurrey and colleagues propose a model in ovarian cancer, in which EMT transcription factors Snail and Slug can antagonize p53-mediated apoptosis (40). TGFβ is also known to simultaneously invoke EMT and block apoptosis via PI3K signaling (45). In addition, another EMT inducer, SIP1, has been ascribed antiapoptotic properties (46). With the acquisition of an EMT phenotype, cells have been shown to increase autophagy: a lysosomal degradation pathway that can be used to increase survival of cells (47). Rouschop and colleagues demonstrated that inhibition of autophagy sensitized xenografts to irradiation (48).
In an attempt to confirm these hypotheses, we tested different gene sets for reactive oxygen species, DNA repair, cell-cycle phase, and several means of cell death against the EMT gene set (Table 4.4). From these analyses, it appears that there is no single explanation for the radioresistance of the mesenchymal phenotype. The acquisition of a heightened EMT gene expression profile corresponds to a higher expression of genes known to be expressed in G2, genes involved in DNA double-strand break repair and autophagy. This indicates that mesenchymal cells might become more resistant to radiotherapy by prolonging time spent in G2, more efficient double-strand break repair, and the use of autophagy as a possible mechanism to evade cell death. ROS scavenger or apoptosis gene sets showed no correlation with expression of EMT genes.
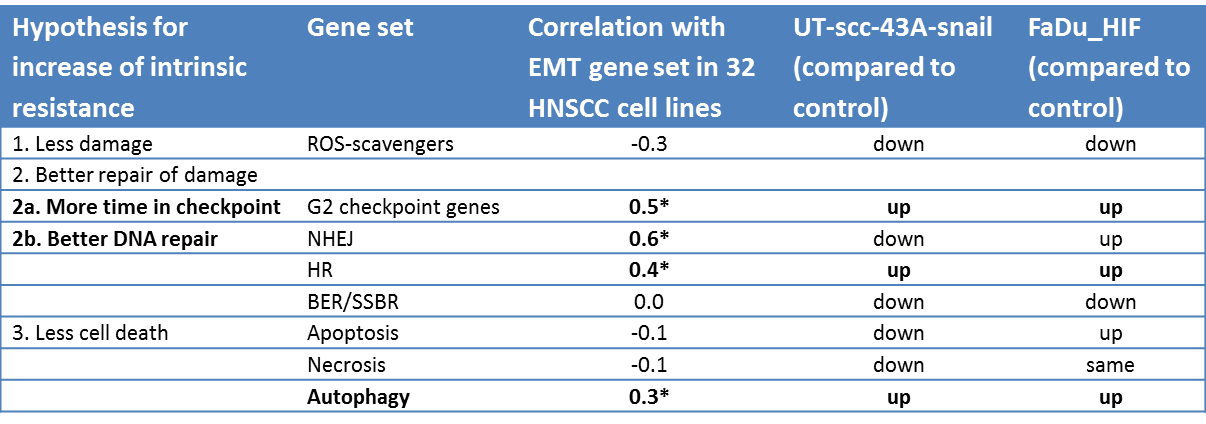
Table 4.4. Results of testing gene sets for reactive oxygen species, DNA repair, cell cycle phase and several means of cell death against the EMT gene set. Spearman’s rank correlations. * p-value <0.05.
Our study is the first to identify miRs with their mRNA targets that are involved in radioresistance in HNSCC. By analyzing miRs together with their targets, a more realistic representation of what occurs in cells can be obtained. A pitfall remains the allocation of the correct targets to every miR. Despite this possible confounding effect of wrongly allocated targets in the analysis, when studying the effect of all targets of one miR as a group, a reliable target effect can be observed. Future studies into correctly defining miR targets should improve this analysis method. The potential advantage of discovering miRs that are correlated with resistance is that, when used as therapeutic agents, they are able to target many genes at once, frequently within one pathway or network (49).
We observed that constitutive but not radiation-responsive genes correlated with radioresistance. These findings are consistent with findings of Birrell and colleagues on the yeast deletion mutant library (50) and the findings in the gene expression series of Amundson and colleagues who concluded that in the NCI-60 cell line panel “basal expression patterns discriminated well between radiosensitive and more resistant lines, possibly being more informative than radiation response signatures” (8).
In conclusion, the pre-irradiation miR-203 status, determined by integrative miR and mRNA analyses, was the most powerful predictor of radioresistance in our HNSCC cell line panel. This EMT-inhibiting miR was decreased in patients with a local recurrence after radiotherapy. The fact that radioresistance could be best predicted from baseline expression suggests that future studies into intrinsic resistance should not focus on response to irradiation. If these findings can be translated to the clinical setting, it should be possible to predict radiotherapy outcome from a pretreatment sample.
The next step would be to reverse EMT in vivo, possibly by restoring expression of miR-203. Because one miR can target many genes, EMT caused via different routes could potentially be inhibited by a single miR. Inhibition of EMT in vivo could not only make cells more radiosensitive but also more chemosensitive and less invasive, which together should lead to better patient survival.